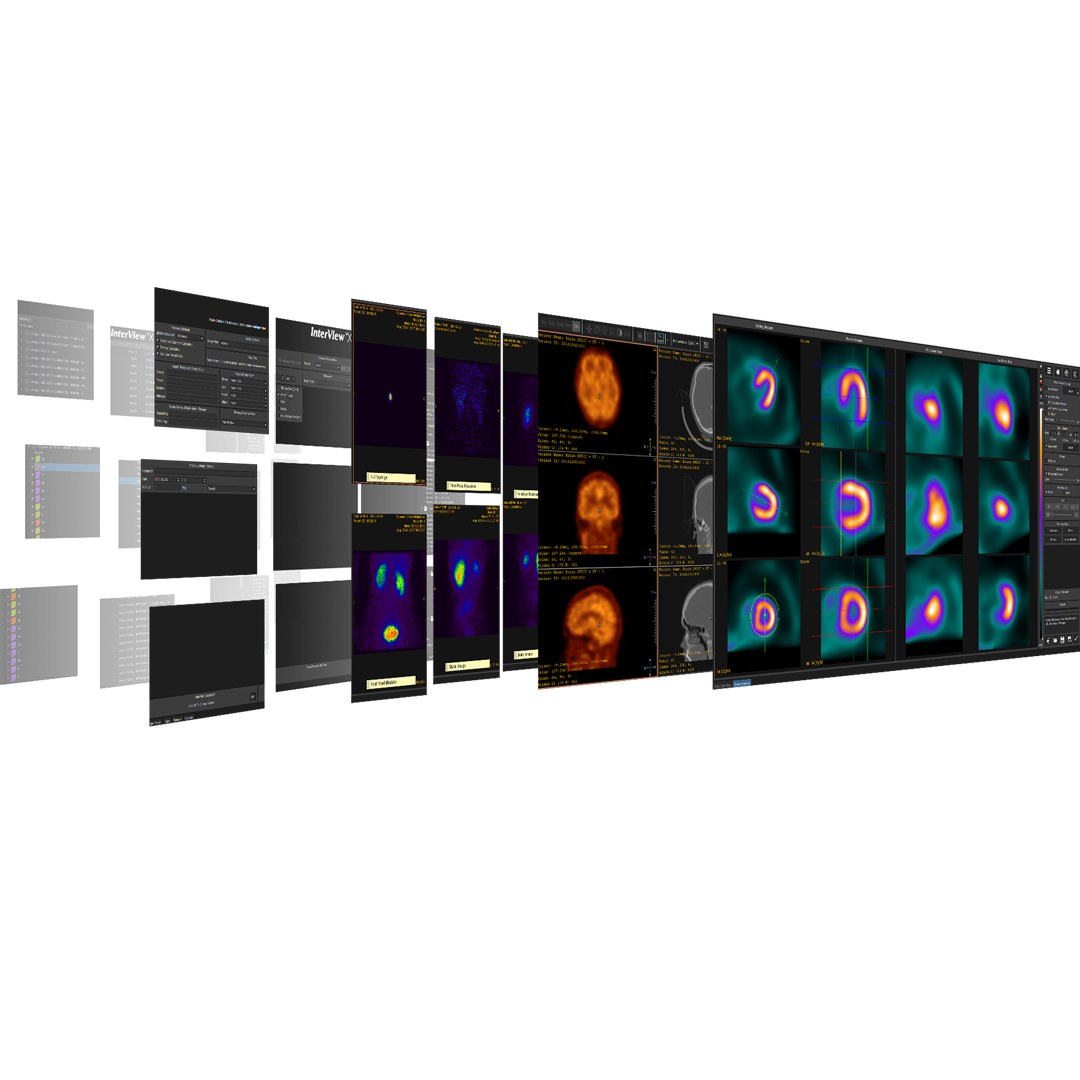
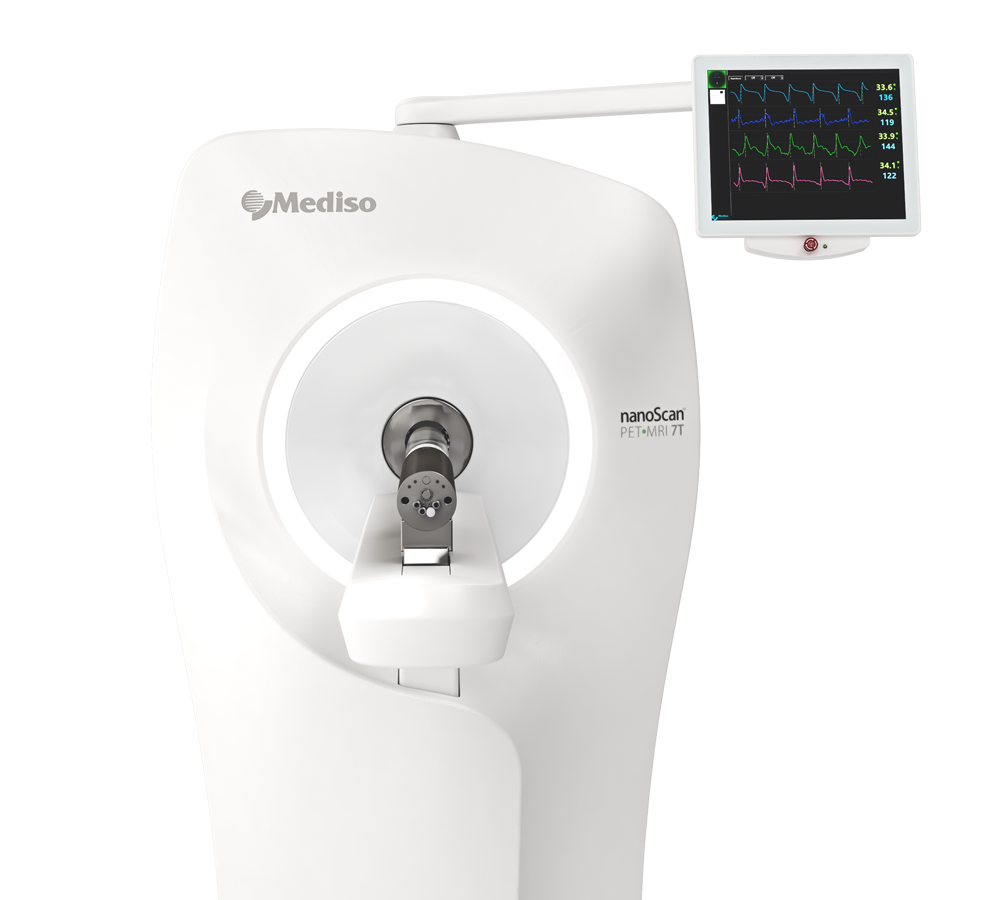
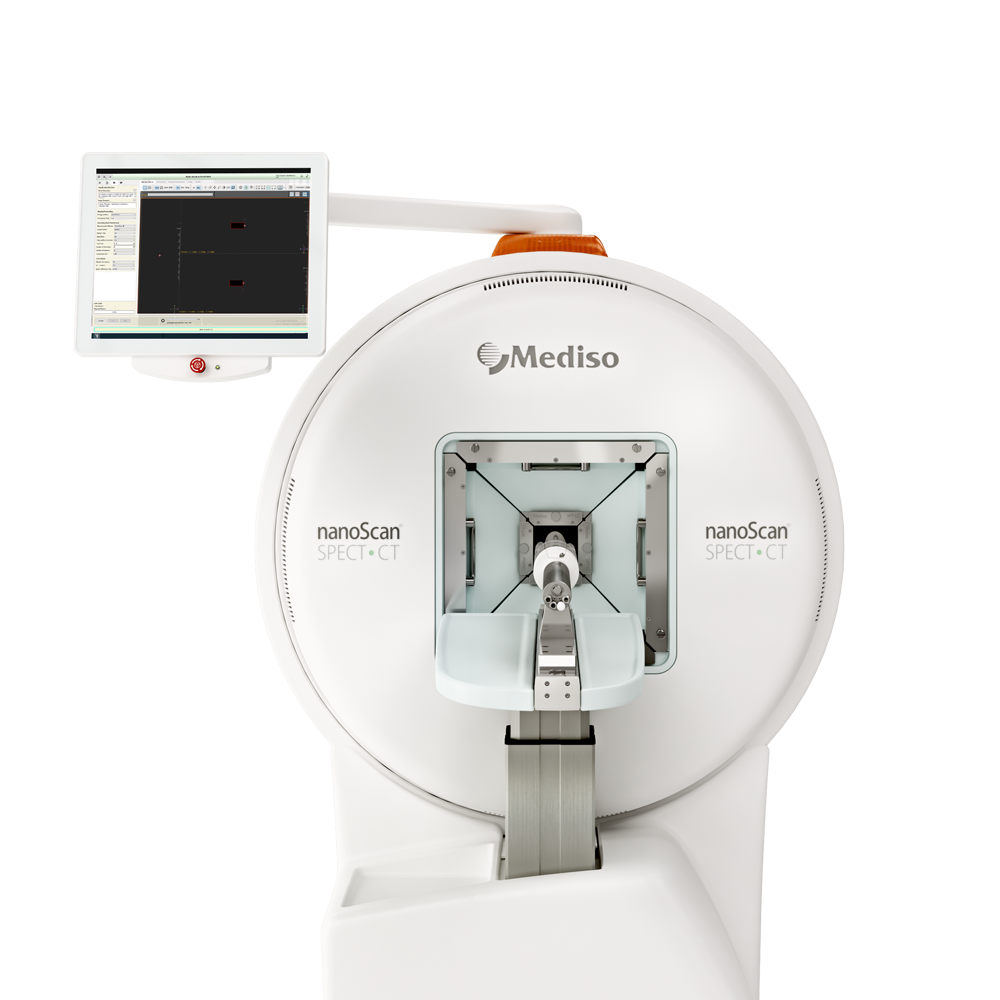
Products suited for your business
Preclinical products
The equipment of Mediso nanoScan® Family deliver the current and future promise of molecular imaging by maximizing functional information in combination with precise anatomical detail.
Latest news & events
About us
Since 1990, Mediso is creating new technologies, and molecular imaging systems.
Clinical and pre-clinical solutions for hospitals, clinics and scientific institution
How can we help you?
Don't hesitate to contact us for technical information or to find out more about our products and services.
Get in touch